Scientific Research
My research spans neuroscience, gene editing, and advanced imaging techniques. Explore my publications, conference presentations, and visual showcase of imaging work.
CNS Gene Therapy
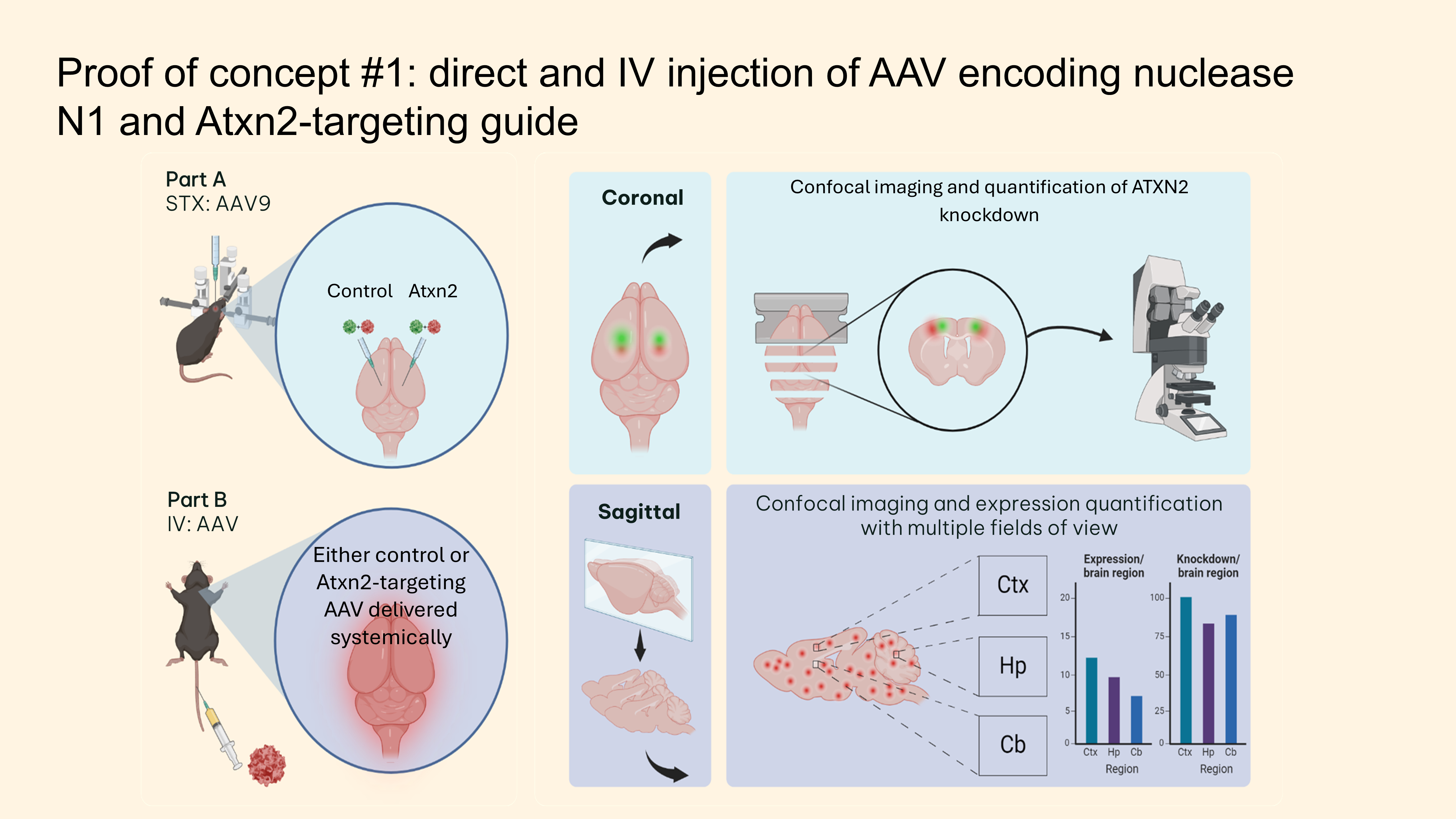
Projects involving Custom stereotactic systems for precise CNS delivery of AAV and LNP, combined with advanced tissue clearing and imaging techniques for biodistribution analysis.
Custom Stereotactic Injection System
- Built custom stereotactic rig for precise AAV delivery to CNS targets
- Optimized injection protocols for various brain regions
- Implemented quality control systems for surgical procedures
- Trained team members on stereotactic techniques
Blood-Brain Barrier Penetrant AAVs
- Utilized engineered AAV capsids for systemic CNS delivery
- Tested multiple AAV serotypes and engineered variants
- Evaluated biodistribution across entire CNS
- Developed assays for knockdown efficacy assessment
Advanced Imaging & Analysis
- Implemented lipid-removal tissue clearing protocols
- Light-sheet microscopy for whole-organ imaging
- 3D cell segmentation and quantification
- Python/Napari-based analysis pipelines
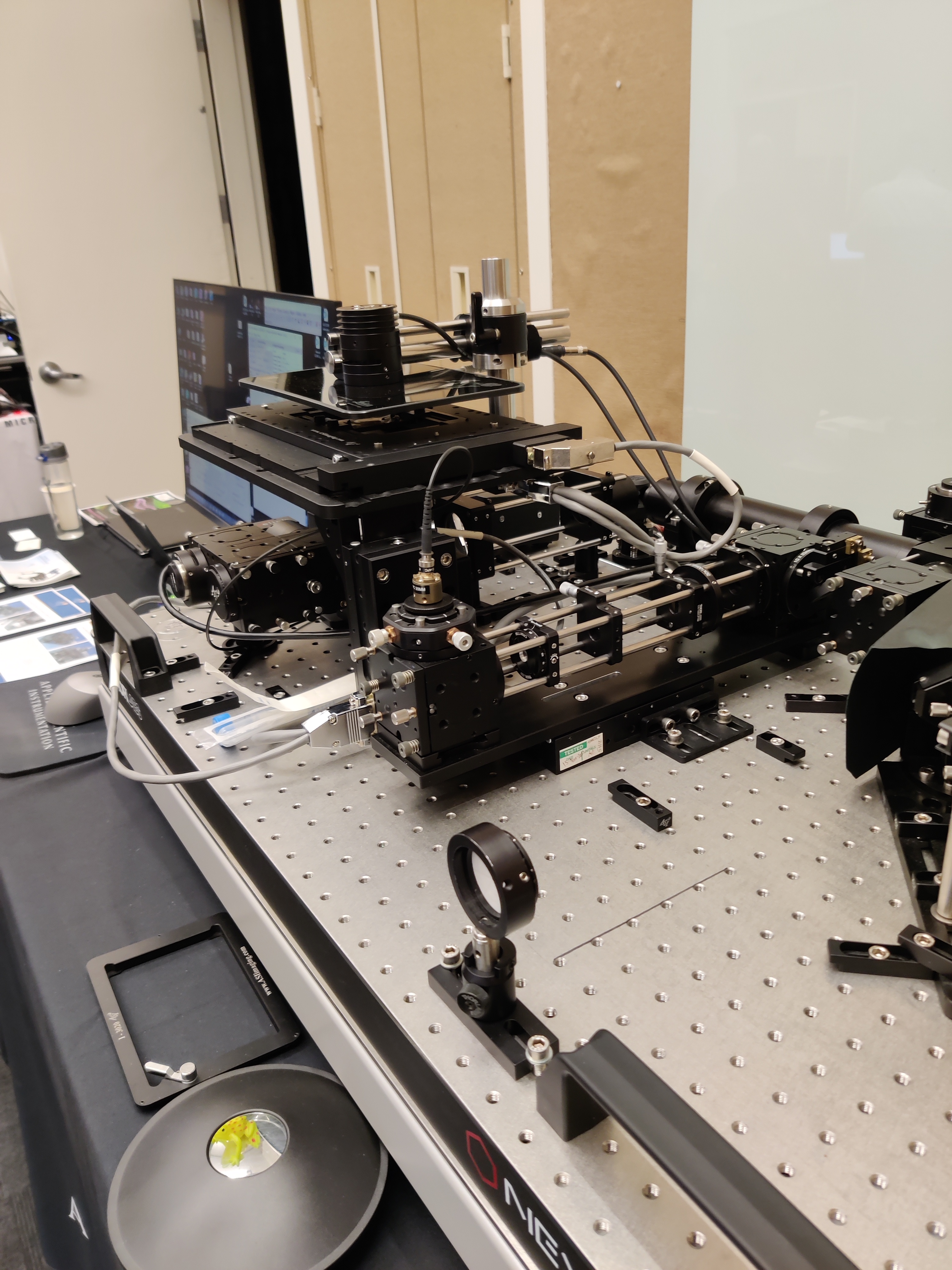
Custom stereotactic surgery suite


Stereotactic system and CLARITY-cleared tissue
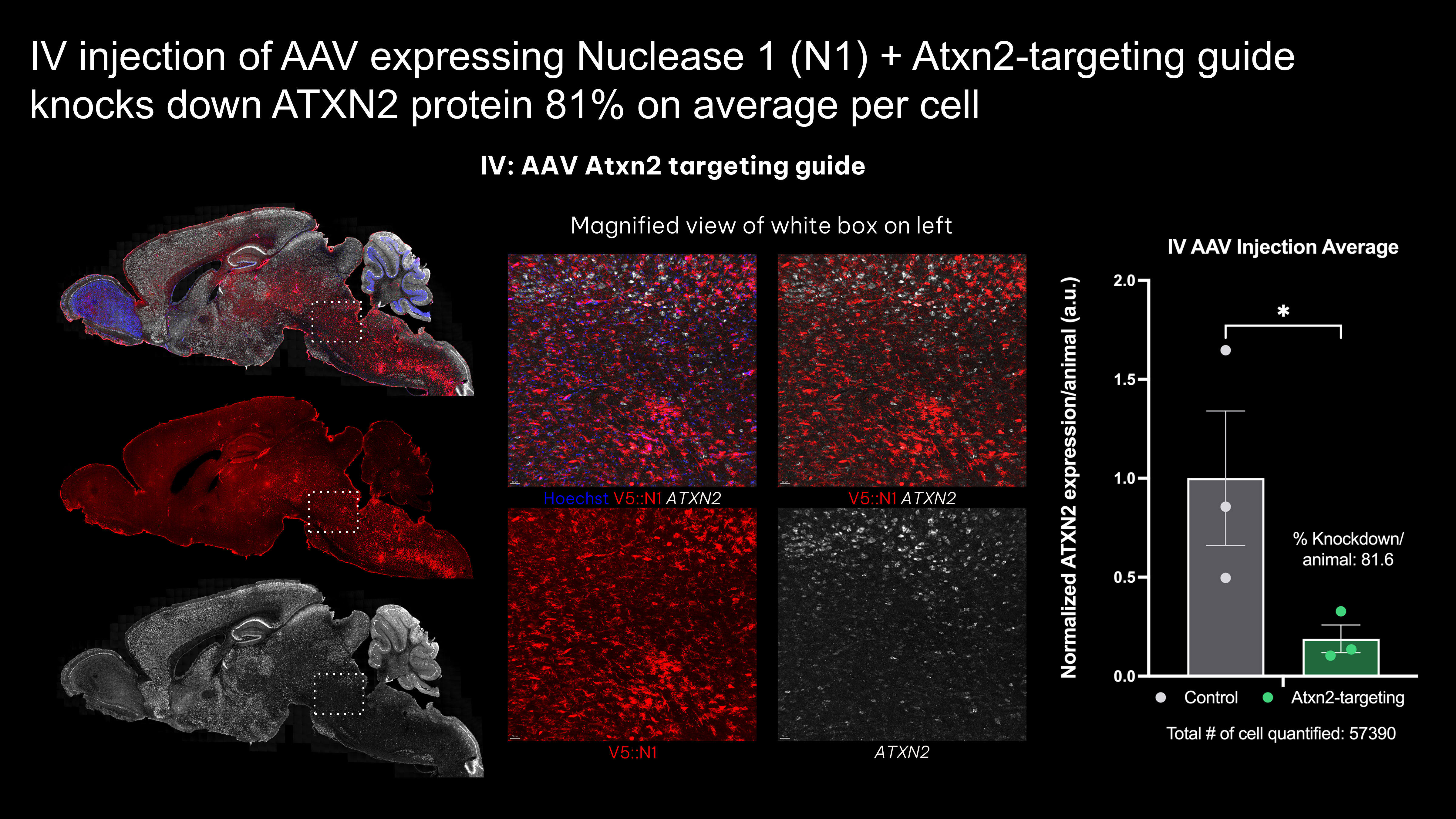
Blood-brain-barrier penetrant AAVs expressing engineered nuclease knockdown ATXN2 protein by up to 81%
Key Insights
- IV injection of all-in-one BBB-penetrant AAVs knocks down ATXN2 protein in vivo
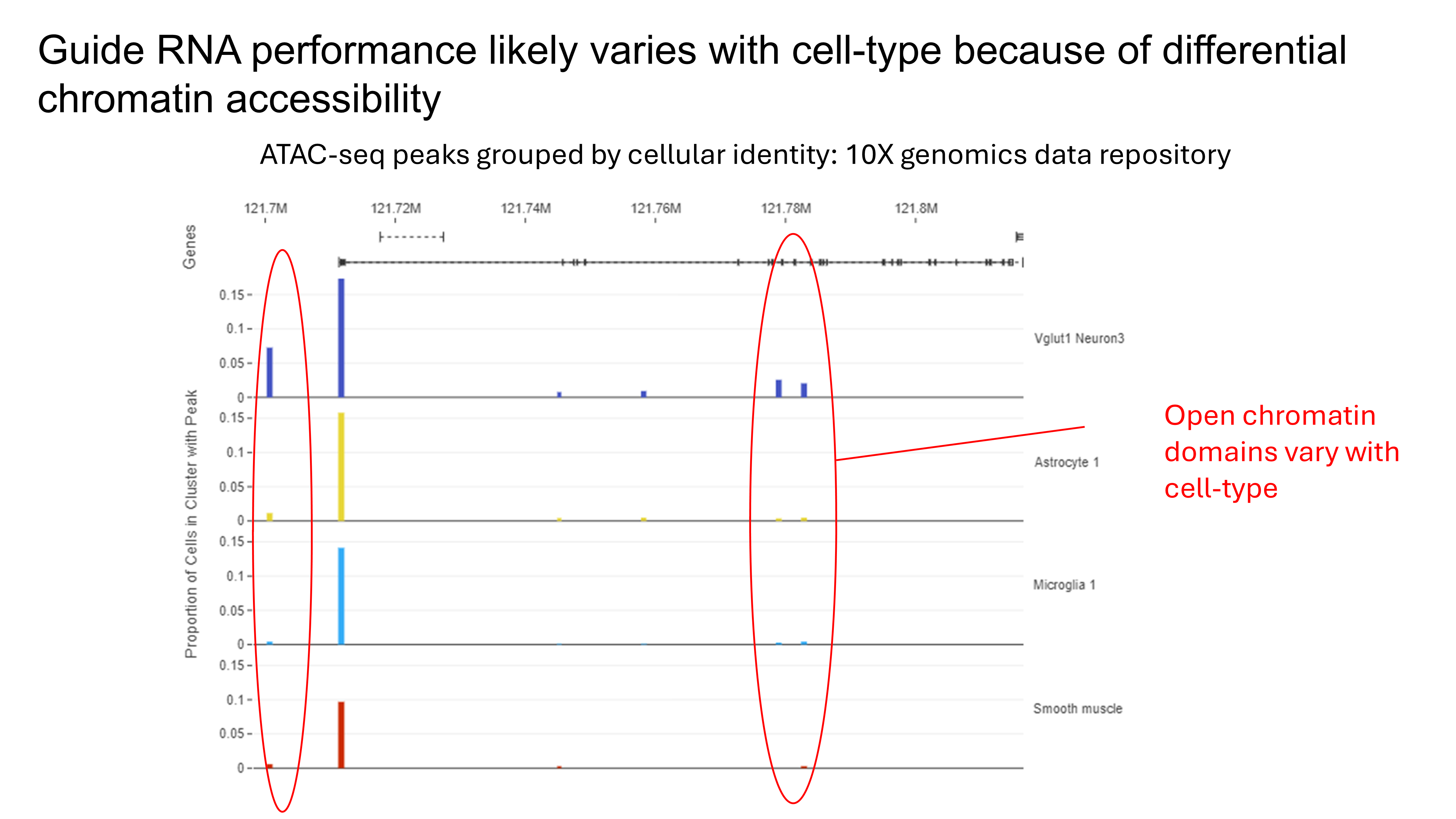
- Efficacy is likely cell-type specific
Key Insights
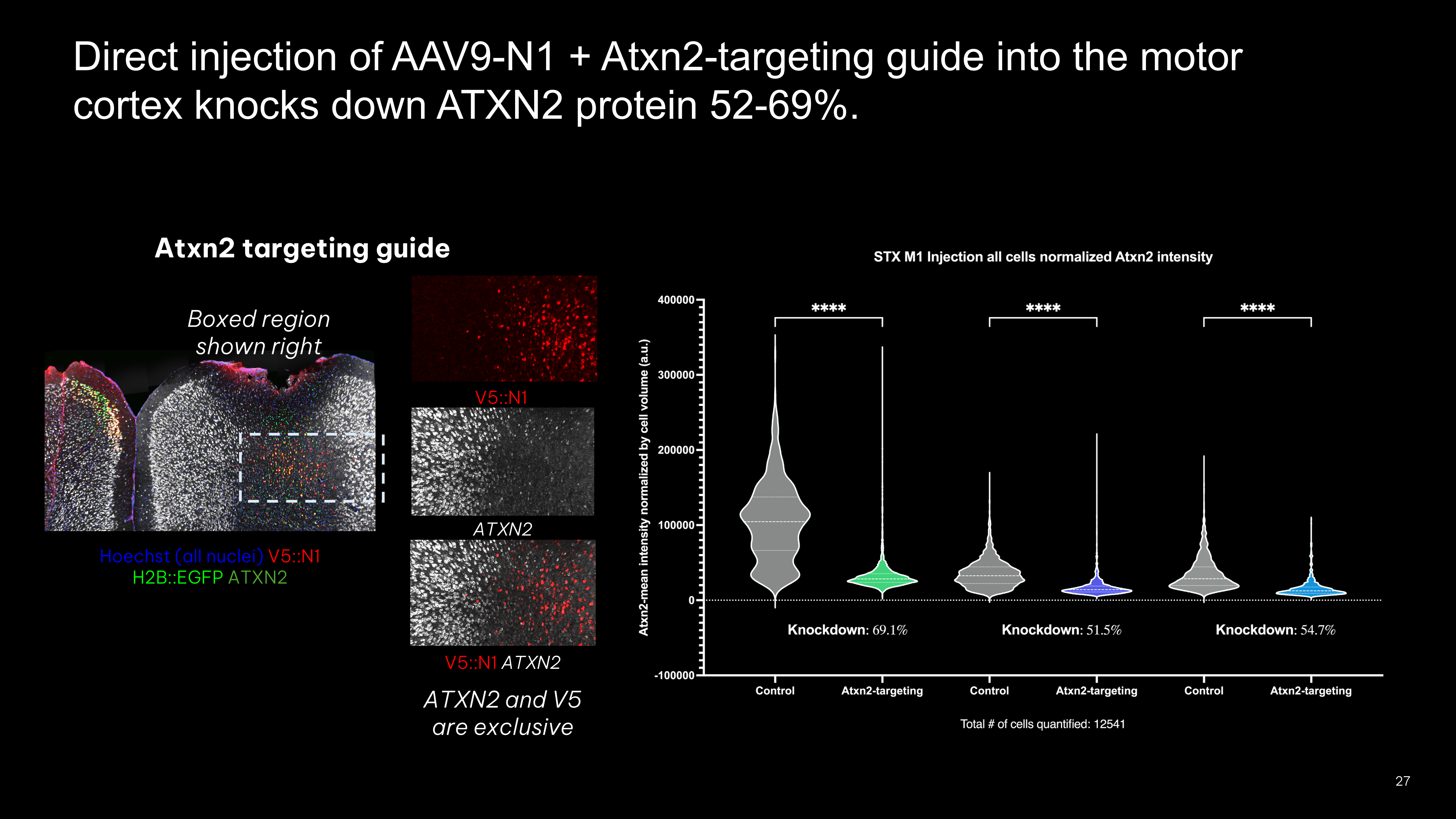
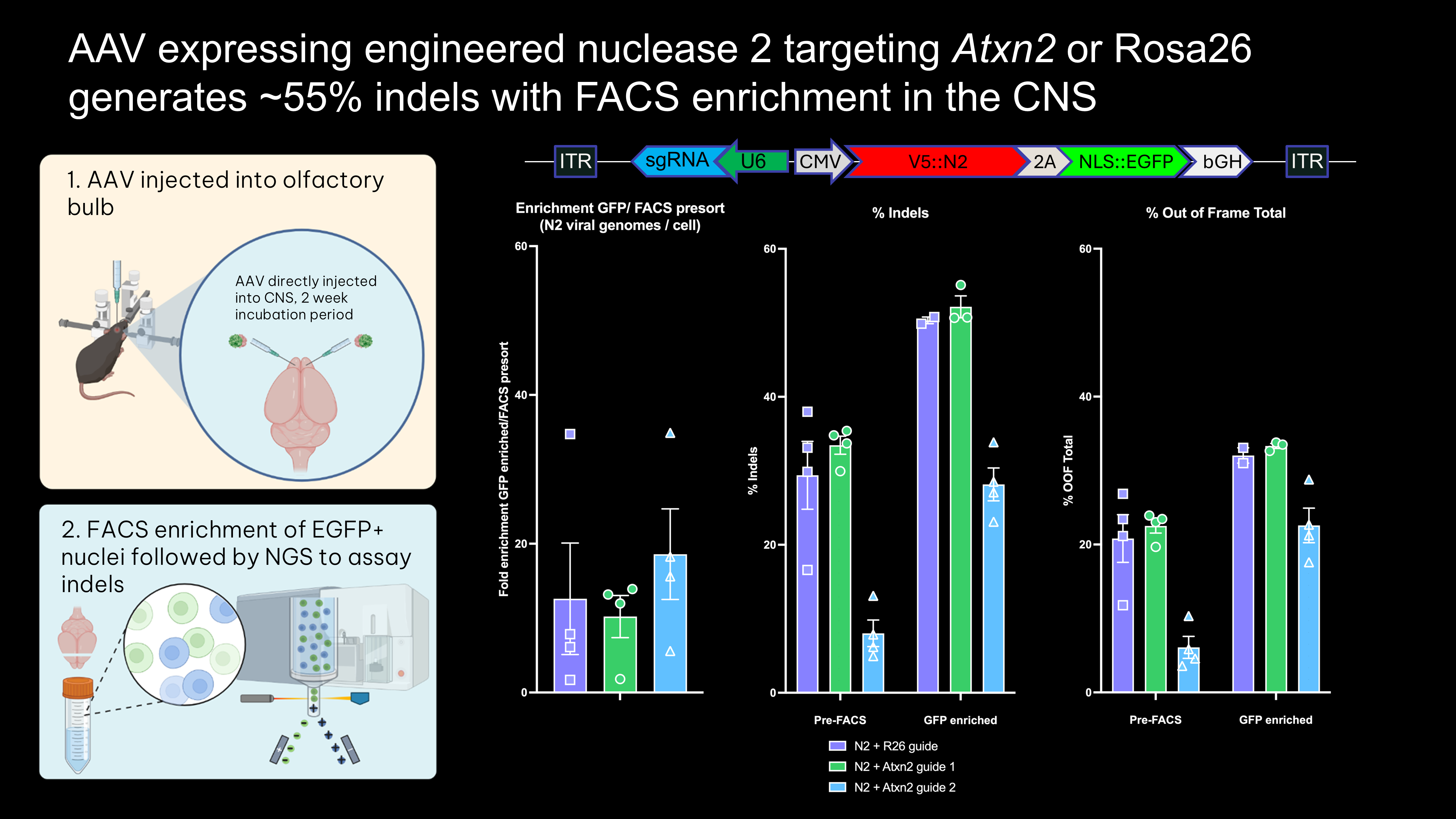
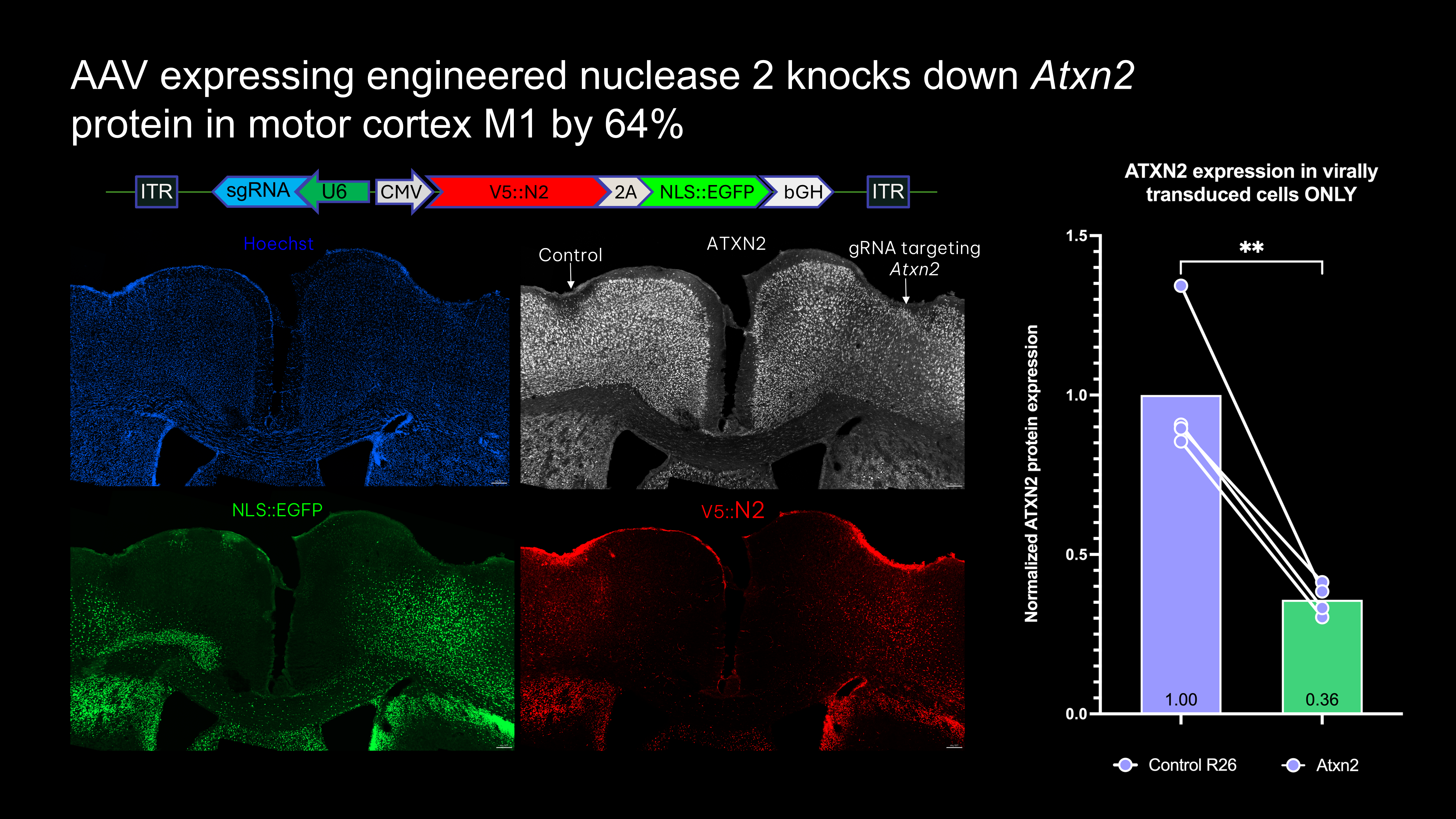
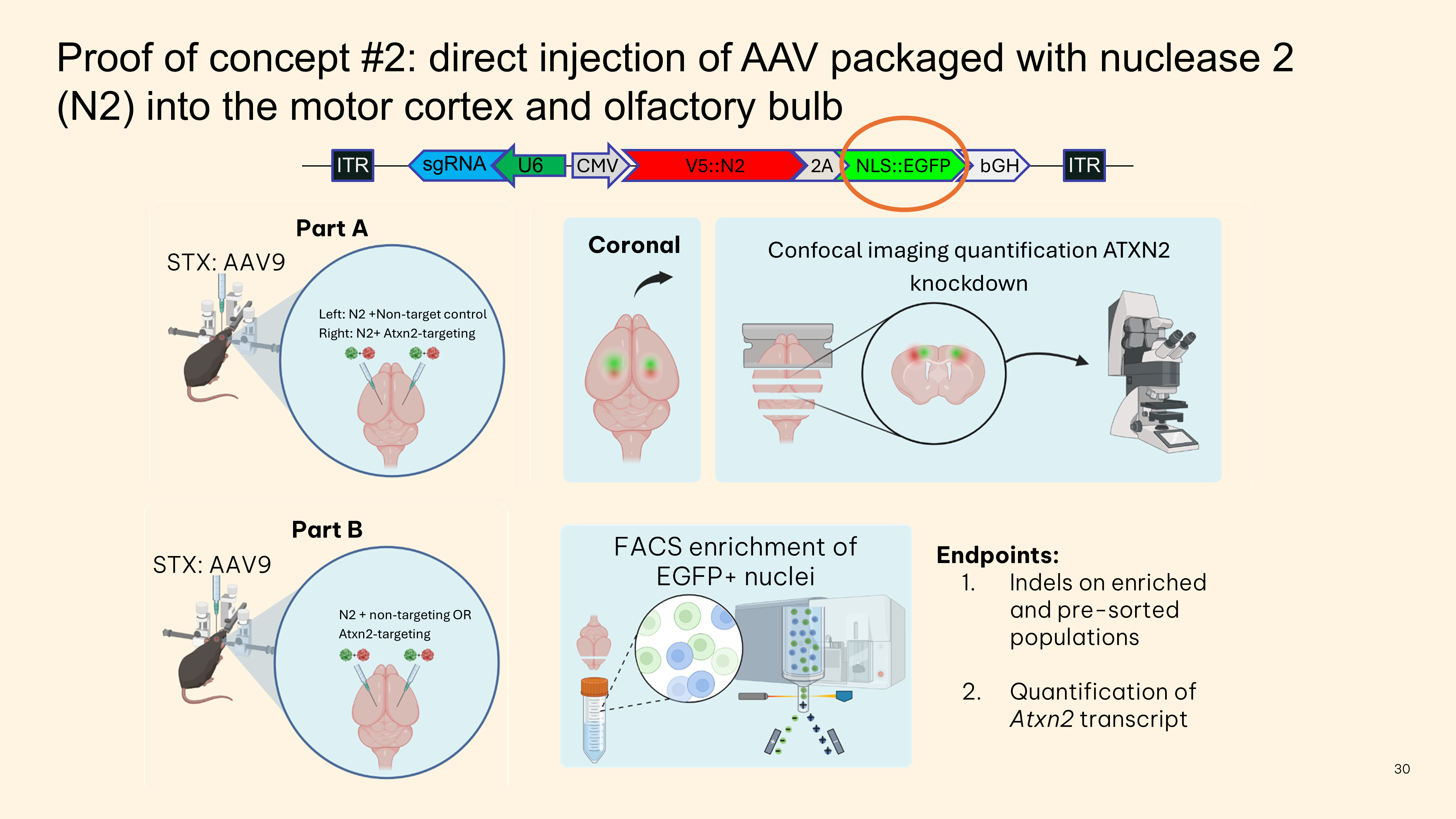
- Direct injection of all-in-one-AAV expressing epitope-tagged nuclease, guide, and fluorophore knocks down ATXN2 protein in vivo
- FACS enrichment using epitope-tag shows that nuclease genome editing efficiency is around 55% in the motor cortex M1 region
Advanced Microscopy & Imaging
Expertise in cutting-edge microscopy techniques for imaging large tissue chunks, traditional sections, organoids, and live cells/organisms. Specializing in light-sheet, two-photon, and confocal microscopy.
MesoSPIM Light-Sheet Imaging of Cleared Whole Brain
Light-Sheet Microscopy
- MesoSPIM whole brain imaging at subcellular resolution
- Cleared tissue visualization with CLARITY and iDISCO protocols
- Large-scale 3D reconstruction and volumetric analysis
- Vascular network mapping in intact organs
Two-Photon & Confocal Imaging
- Deep tissue imaging of neuronal populations
- Live cell imaging and calcium dynamics
- High-resolution structural imaging of brain sections
- Multi-channel fluorescence for circuit mapping
Sample Types
- Large tissue chunks (whole brains, spinal cords)
- Traditional histological sections
- Organoids and 3D cultures
- Live cells and model organisms
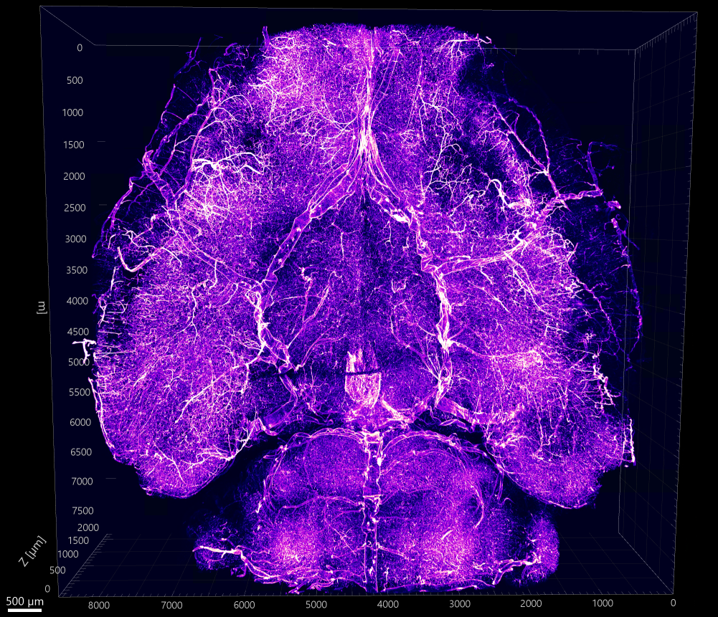
Whole brain vascular network visualization
Intact Cleared Tissue Imaging
Confocal imaging: AAV-labeled neurons in olfactory bulb
Two-photon imaging: Olfactory bulb neuronal populations
Segmentation & Computational Analysis
Advanced image processing and quantification using commercial software and custom Python pipelines for handling terabyte-scale datasets and extracting meaningful biological insights.
Software Expertise
- Imaris: 3D cell segmentation and tracking, surface rendering, filament tracing
- Arivis: Large dataset visualization, automated batch processing, object classification
- Fiji/ImageJ: Custom macro development, plugin integration, automated workflows
- Python/Napari: Custom analysis pipelines for terabyte-scale data, GPU acceleration
Analysis Capabilities
- 3D cell segmentation and morphology quantification
- Automated cell counting and density mapping
- Fiber/neurite tracing and connectivity analysis
- Multi-region brain quantification
- Statistical analysis and data visualization
- High-throughput batch processing pipelines
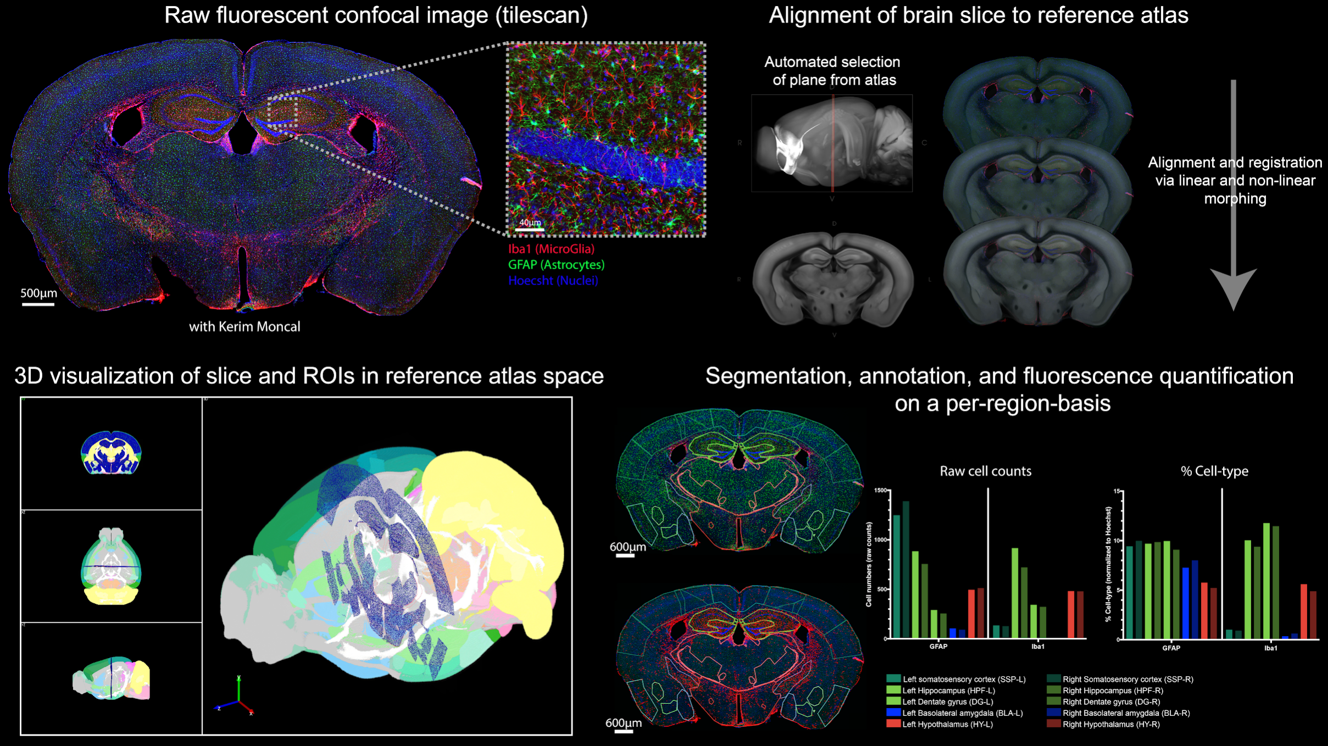
Automated brain slice alignment for whole organ quantification
Whole Organ Quantification
Spinal cord motor neuron quantification - Automated segmentation and counting across entire spinal cord sections
Tissue Section Quantification
Automated 3D cell segmentation - High-throughput quantification of cellular populations in tissue sections
3D neuronal morphology reconstruction
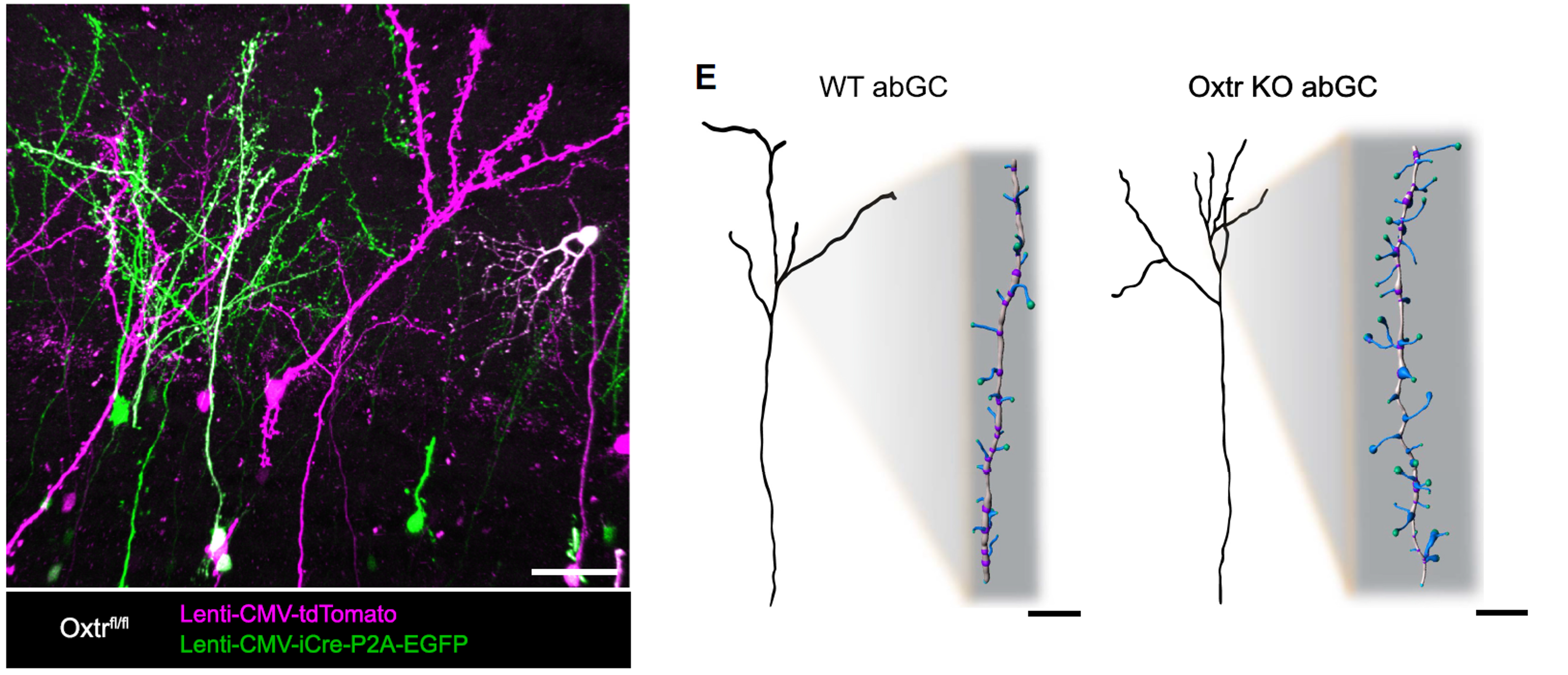
Morphology analysis
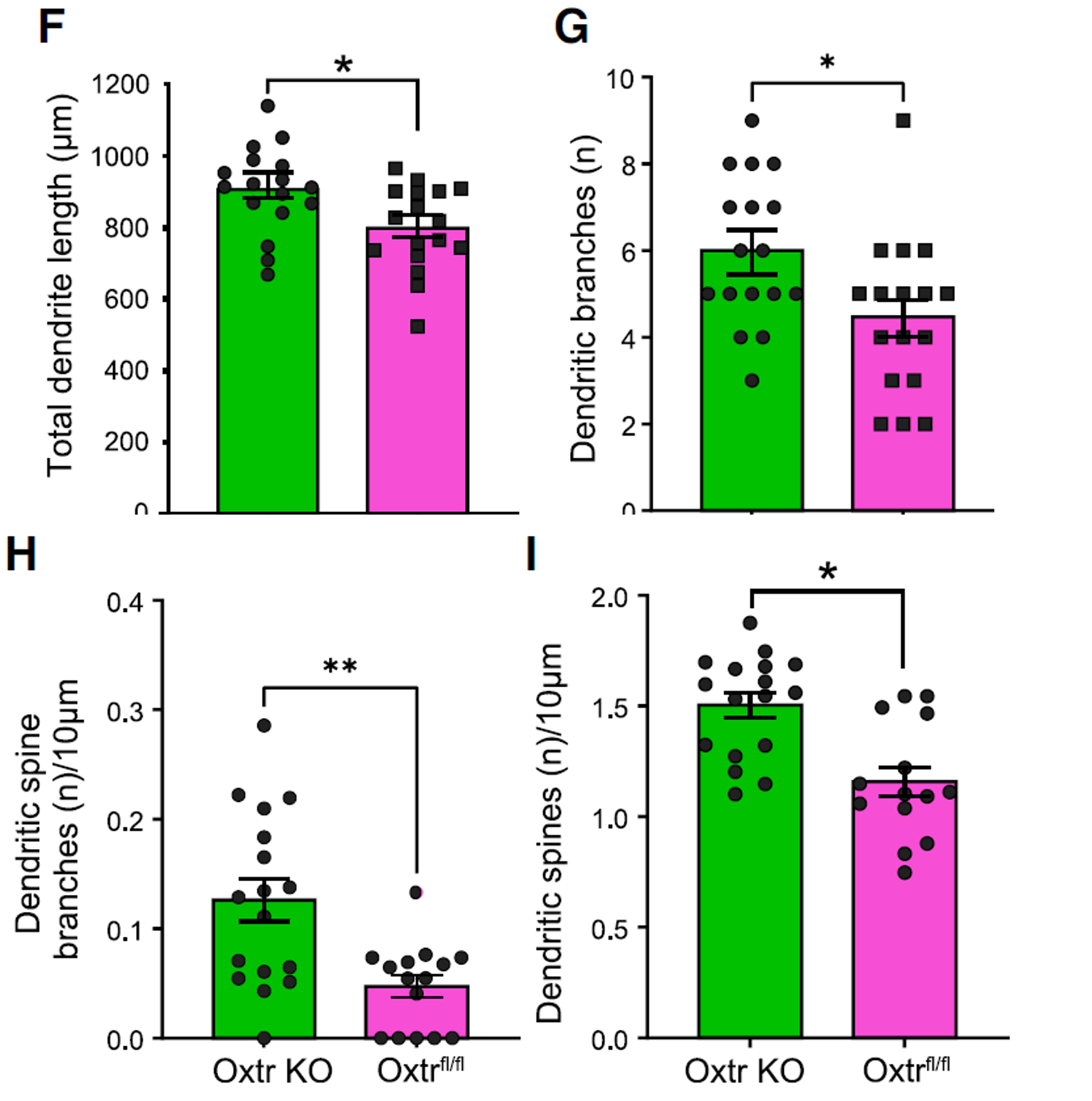
Statistical results
Recent Conferences
Society for Neuroscience 2025
San Diego | November 2025

Advanced Imaging Methods Conference
UC Berkeley | January 21-23, 2025
Invited Speaker
Title: Imaging methods for interrogating gene editing in the CNS
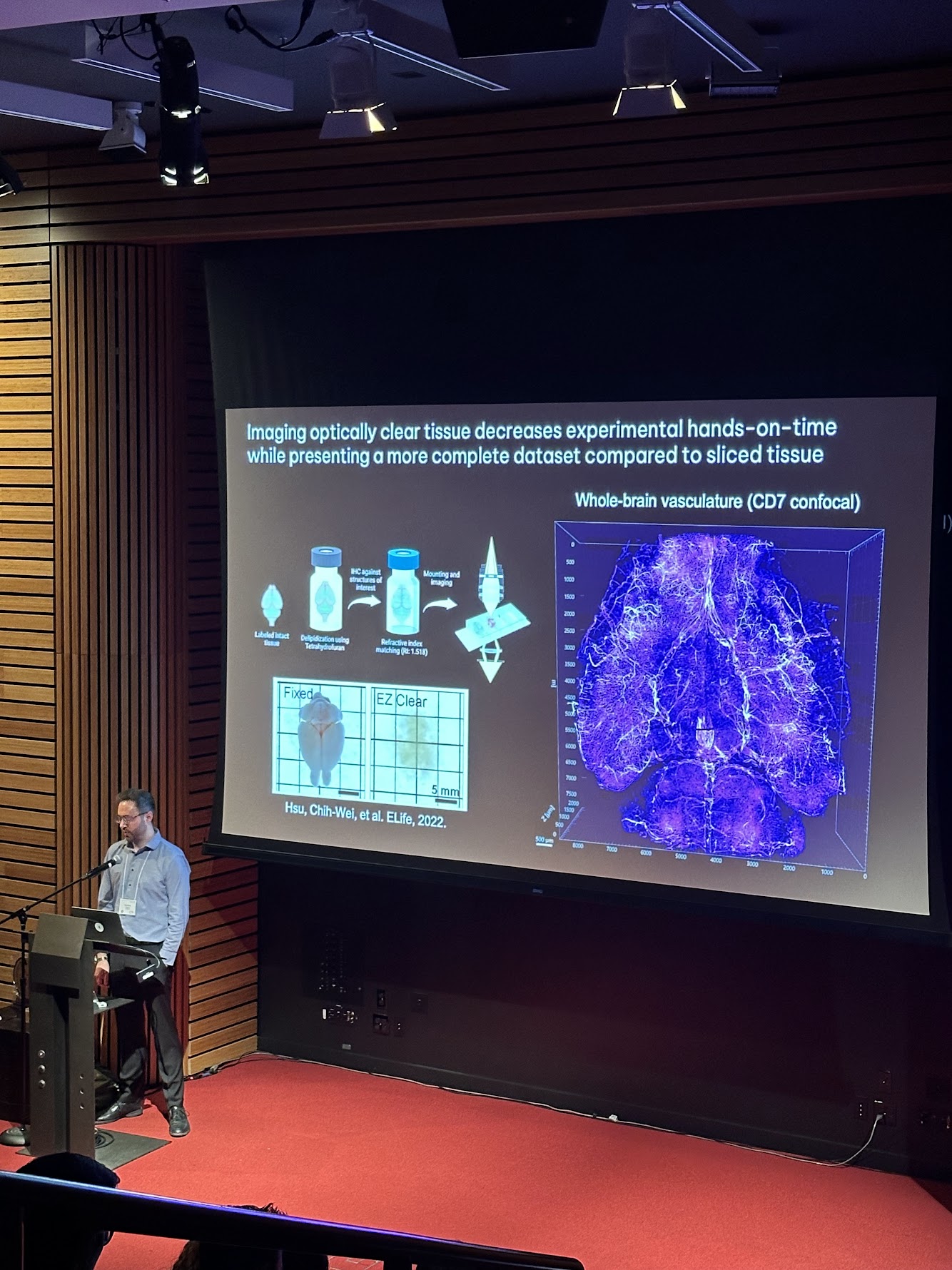
American Society of Gene and Cell Therapy (ASGCT) 2025
2025
Poster 1: A Compact and Potent Type II CRISPR System for CNS Gene Knockdown via AAV Delivery
Poster 2: In Vivo Genome Editing with an Ultra-Compact Type V Nuclease for All-In-One AAV Delivery
Click to view full poster
Publications
Single-cell RNA-seq of mouse olfactory bulb reveals cellular heterogeneity and activity-dependent molecular census of adult-born neurons
Cell reports 25 (10), 2689-2703. e3, 2018 (2018)
Abstract
Cellular heterogeneity within the mammalian brain poses a challenge toward understanding its complex functions. Within the olfactory bulb, odor information is processed by subtypes of inhibitory interneurons whose heterogeneity and functionality are influenced by ongoing adult neurogenesis. To investigate this cellular heterogeneity and better understand adult-born neuron development, we utilized single-cell RNA sequencing and computational modeling to reveal diverse and transcriptionally distinct neuronal and nonneuronal cell types. We also analyzed molecular changes during adult-born interneuron maturation and uncovered developmental programs within their gene expression profiles. Finally, we identified that distinct neuronal subtypes are differentially affected by sensory experience. Together, these data provide a transcriptome-based foundation for investigating subtype-specific neuronal function in …
Olfactory bulb astrocytes mediate sensory circuit processing through Sox9 in the mouse brain
Nature communications 12 (1), 5230, 2021 (2021)
Abstract
The role of transcription factors during astrocyte development and their subsequent effects on neuronal development has been well studied. Less is known about astrocytes contributions towards circuits and behavior in the adult brain. Astrocytes play important roles in synaptic development and modulation, however their contributions towards neuronal sensory function and maintenance of neuronal circuit architecture remain unclear. Here, we show that loss of the transcription factor Sox9 results in both anatomical and functional changes in adult mouse olfactory bulb (OB) astrocytes, affecting sensory processing. Indeed, astrocyte-specific deletion of Sox9 in the OB results in decreased odor detection thresholds and discrimination and it is associated with aberrant neuronal sensory response maps. At functional level, loss of astrocytic Sox9 impairs the electrophysiological properties of mitral and tufted neurons. RNA …
Oxytocin and sensory network plasticity
Frontiers in Neuroscience 14, 514782, 2020 (2020)
Abstract
An essential characteristic of nervous systems is their capacity to reshape functional connectivity in response to physiological and environmental cues. Endogenous signals, including neuropeptides, governs nervous system plasticity. Particularly, oxytocin has been recognized for its role in mediating activity-dependent circuit changes. These oxytocin-dependent changes occur at the synaptic level and consequently shape the cellular composition of circuits. Here we discuss recent advances that illustrate how oxytocin functions to reshape neural circuitry in response to environmental changes. Excitingly, recent findings pave the way for promising therapeutic applications of oxytocin to treat neurodevelopmental and neuropsychiatric diseases.
Parallel astrocyte calcium signaling modulates olfactory bulb responses
Journal of neuroscience research 98 (8), 1605-1618, 2020 (2020)
Abstract
Astrocytes are the most abundant glial cell in the central nervous system. They modulate synaptic function through a variety of mechanisms, and yet remain relatively understudied with respect to overall neuronal circuit function. Exploiting the tractability of the mouse olfactory system, we manipulated astrocyte activity and examined how astrocytes modulate olfactory bulb responses. Toward this, we genetically targeted both astrocytes and neurons for in vivo widefield imaging of Ca2+ responses to odor stimuli. We found that astrocytes exhibited odor response maps that overlap with excitatory neuronal activity. By manipulating Ca2+ activity in astrocytes using chemical genetics we found that odor‐evoked neuronal activity was reciprocally affected, suggesting that astrocyte activation inhibits neuronal odor responses. Subsequently, behavioral experiments revealed that astrocyte manipulations affect both odor …
Target specific functions of EPL interneurons in olfactory circuits
Nature Communications 10 (1), 3369, 2019 (2019)
Abstract
Inhibitory interneurons are integral to sensory processing, yet revealing their cell type-specific roles in sensory circuits remains an ongoing focus. To Investigate the mouse olfactory system, we selectively remove GABAergic transmission from a subset of olfactory bulb interneurons, EPL interneurons (EPL-INs), and assay odor responses from their downstream synaptic partners — tufted cells and mitral cells. Using a combination of in vivo electrophysiological and imaging analyses, we find that inactivating this single node of inhibition leads to differential effects in magnitude, reliability, tuning width, and temporal dynamics between the two principal neurons. Furthermore, tufted and not mitral cell responses to odor mixtures become more linearly predictable without EPL-IN inhibition. Our data suggest that olfactory bulb interneurons, through exerting distinct inhibitory functions onto their different synaptic partners, play a …
POU6f1 mediates neuropeptide-dependent plasticity in the adult brain
Journal of Neuroscience 38 (6), 1443-1461, 2018 (2018)
Abstract
The mouse olfactory bulb (OB) features continued, activity-dependent integration of adult-born neurons, providing a robust model with which to examine mechanisms of plasticity in the adult brain. We previously reported that local OB interneurons secrete the neuropeptide corticotropin-releasing hormone (CRH) in an activity-dependent manner onto adult-born granule neurons and that local CRH signaling promotes expression of synaptic machinery in the bulb. This effect is mediated via activation of the CRH receptor 1 (CRHR1), which is developmentally regulated during adult-born neuron maturation. CRHR1 is a GS-protein-coupled receptor that activates CREB-dependent transcription in the presence of CRH. Therefore, we hypothesized that locally secreted CRH activates CRHR1 to initiate circuit plasticity programs. To identify such programs, we profiled gene expression changes associated with CRHR1 …
Activation of basal forebrain-to-lateral habenula circuitry drives reflexive aversion and suppresses feeding behavior
Scientific reports 12 (1), 22044, 2022 (2022)
Abstract
Environmental cues and internal states such as mood, reward, or aversion directly influence feeding behaviors beyond homeostatic necessity. The hypothalamus has been extensively investigated for its role in homeostatic feeding. However, many of the neural circuits that drive more complex, non-homeostatic feeding that integrate valence and sensory cues (such as taste and smell) remain unknown. Here, we describe a basal forebrain (BF)-to-lateral habenula (LHb) circuit that directly modulates non-homeostatic feeding behavior. Using viral-mediated circuit mapping, we identified a population of glutamatergic neurons within the BF that project to the LHb, which responds to diverse sensory cues, including aversive and food-related odors. Optogenetic activation of BF-to-LHb circuitry drives robust, reflexive-like aversion. Furthermore, activation of this circuitry suppresses the drive to eat in a fasted state. Together …
Oxytocin signaling is necessary for synaptic maturation of adult-born neurons
Genes & Development 36 (21-24), 1100-1118, 2022 (2022)
Abstract
Neural circuit plasticity and sensory response dynamics depend on forming new synaptic connections. Despite recent advances toward understanding the consequences of circuit plasticity, the mechanisms driving circuit plasticity are unknown. Adult-born neurons within the olfactory bulb have proven to be a powerful model for studying circuit plasticity, providing a broad and accessible avenue into neuron development, migration, and circuit integration. We and others have shown that efficient adult-born neuron circuit integration hinges on presynaptic activity in the form of diverse signaling peptides. Here, we demonstrate a novel oxytocin-dependent mechanism of adult-born neuron synaptic maturation and circuit integration. We reveal spatial and temporal enrichment of oxytocin receptor expression within adult-born neurons in the murine olfactory bulb, with oxytocin receptor expression peaking during activity …
Tau polarizes an aging transcriptional signature to excitatory neurons and glia
Elife 12, e85251, 2023 (2023)
Abstract
Aging is a major risk factor for Alzheimer’s disease (AD), and cell-type vulnerability underlies its characteristic clinical manifestations. We have performed longitudinal, single-cell RNA-sequencing in Drosophila with pan-neuronal expression of human tau, which forms AD neurofibrillary tangle pathology. Whereas tau-and aging-induced gene expression strongly overlap (93%), they differ in the affected cell types. In contrast to the broad impact of aging, tau-triggered changes are strongly polarized to excitatory neurons and glia. Further, tau can either activate or suppress innate immune gene expression signatures in a cell-type-specific manner. Integration of cellular abundance and gene expression pinpoints nuclear factor kappa B signaling in neurons as a marker for cellular vulnerability. We also highlight the conservation of cell-type-specific transcriptional patterns between Drosophila and human postmortem brain tissue. Overall, our results create a resource for dissection of dynamic, age-dependent gene expression changes at cellular resolution in a genetically tractable model of tauopathy.
Co-transmitting neurons in the lateral septal nucleus exhibit features of neurotransmitter switching
IBRO neuroscience reports 12, 390-398, 2022 (2022)
Abstract
No abstract available.
Olfactory bulb astrocytes mediate sensory circuit processing through Sox9 in the mouse brain. Nat. Commun. 12, 5230
(2021)
Abstract
No abstract available.
Olfactory cued learning paradigm
Bio-protocol 7 (9), e2251-e2251, 2017 (2017)
Abstract
Sensory stimulation leads to structural changes within the CNS (Central Nervous System), thus providing the fundamental mechanism for learning and memory. The olfactory circuit offers a unique model for studying experience-dependent plasticity, partly due to a continuous supply of integrating adult born neurons. Our lab has recently implemented an olfactory cued learning paradigm in which specific odor pairs are coupled to either a reward or punishment to study downstream circuit changes. The following protocol outlines the basic set up for our learning paradigm. Here, we describe the equipment setup, programming of software, and method of behavioral training.
Comparative analysis of AAV serotypes for transduction of olfactory sensory neurons
Frontiers in Neuroscience 19, 1531122, 2025 (2025)
Abstract
Olfactory sensory neurons within the nasal epithelium detect volatile odorants and relay odor information to the central nervous system. Unlike other sensory inputs, olfactory sensory neurons interface with the external environment and project their axons directly into the central nervous system. The use of adeno-associated viruses to target these neurons has garnered interest for applications in gene therapy, probing olfactory sensory neuron biology, and modeling disease. To date, there is no consensus on the optimal AAV serotype for efficient and selective transduction of olfactory sensory neurons in vivo. Here we utilized serial confocal imaging and single-nucleus RNA sequencing to evaluate the efficacy of 11 different AAV serotypes in transducing murine olfactory sensory neurons via non-invasive nasal inoculation. Our results reveal that AAV1, while highly effective, exhibited broad tropism, whereas AAV-DJ/8 showed the greatest specificity for olfactory sensory neurons.
Imaging and quantification of intact neuronal dendrites via CLARITY tissue clearing
Journal of visualized experiments: JoVE, 10.3791/62532, 2021 (2021)
Abstract
No abstract available.
A Novel Type II CRISPR System Small Enough to Fit in a Single AAV Enables Targeted Gene Knockdown in the CNS
MOLECULAR THERAPY 33 (4), 2025 (2025)
Abstract
No abstract available.
In Vivo Genome Editing with an Ultra-Compact Type V Nuclease for All-In-One AAV Delivery
MOLECULAR THERAPY 33 (4), 2025 (2025)
Abstract
No abstract available.
The Alzheimer's disease risk gene CD2AP modulates mammalian synaptic structure and plasticity
Alzheimer's & Dementia 17, e049854, 2021 (2021)
Abstract
CD2‐Associated protein (CD2AP) is associated with increased risk of late‐onset Alzheimer’s disease. We have previously shown that loss of CD2AP’s Drosophila homolog, cindr, exacerbates Tau toxicity and impairs synaptic maturation and function at the neuromuscular junction. However, little is understood about CD2AP at the central mammalian synapse. The present study demonstrates conservation of CD2AP function at the pre‐synapse from fly to mouse, as well as characterizes CD2AP’s role in maintaining post‐synaptic structure and function.
POU6f1 Mediates Neuropeptide-Dependent Plasticity in the Adult Brain.
Journal of Neuroscience 38 (6), 2018 (2018)
Abstract
The mouse olfactory bulb (OB) features continued, activity-dependent integration of adult-born neurons, providing a robust model with which to examine mechanisms of plasticity in the adult brain. We previously reported that local OB interneurons secrete the neuropeptide corticotropin-releasing hormone (CRH) in an activity-dependent manner onto adult-born granule neurons and that local CRH signaling promotes expression of synaptic machinery in the bulb. This effect is mediated via activation of the CRH receptor 1 (CRHR1), which is developmentally regulated during adult-born neuron maturation. CRHR1 is a Gs-protein coupled receptor that activates CREB-dependent transcription in the presence of CRH. Therefore, we hypothesized that locally secreted CRH activates CRHR1 to initiate circuit plasticity programs. To identify such programs, we profiled gene expression changes associated with CRHR1 activity …
A Combinatorial Approach to Circuit Mapping in the Mouse Olfactory Bulb
Extracellular Recording Approaches, 129-142, 2017 (2017)
Abstract
Neurons form neural circuits through functional synapses. Inputs and outputs across these circuits contribute to the brain’s intricate mechanisms of information processing and behavioral responses. By combining acousto-optic deflector-based scanning microscopy with optogenetics, whole cell electrophysiological recordings, and transgenic viral delivery, researchers can now investigate and map relevant neural circuits with high spatial and cell type-specific precision. The goal of this review is to provide the reader with guidelines and methods for using a combinatorial approach toward circuit mapping in rodent brain tissue.
Interested in Collaboration?
I offer consulting services in imaging, analysis, and gene therapy techniques.
Explore Consulting Services